Sarcoma TumorGraft3D Screen
Introducing the Champions Sarcoma TumorGraft3D Screen, which includes 11 distinct sarcoma models. These models are thoroughly characterized with patient response data, clinical annotations, proliferation status, and responses to standard of care (SOC) treatments.
This screen uses Champions' innovative TumorGraft3D Platform: a unique matrix-free assay using indication-specific proprietary media to allow for TumorGraft3D formation and maintenance. This assay can be used to rank order of the effect of different test agents across multiple models, investigate tumor cell biology and interaction with the tumor microenvironment, and assess synergy of therapeutic agents in experimental combinations.
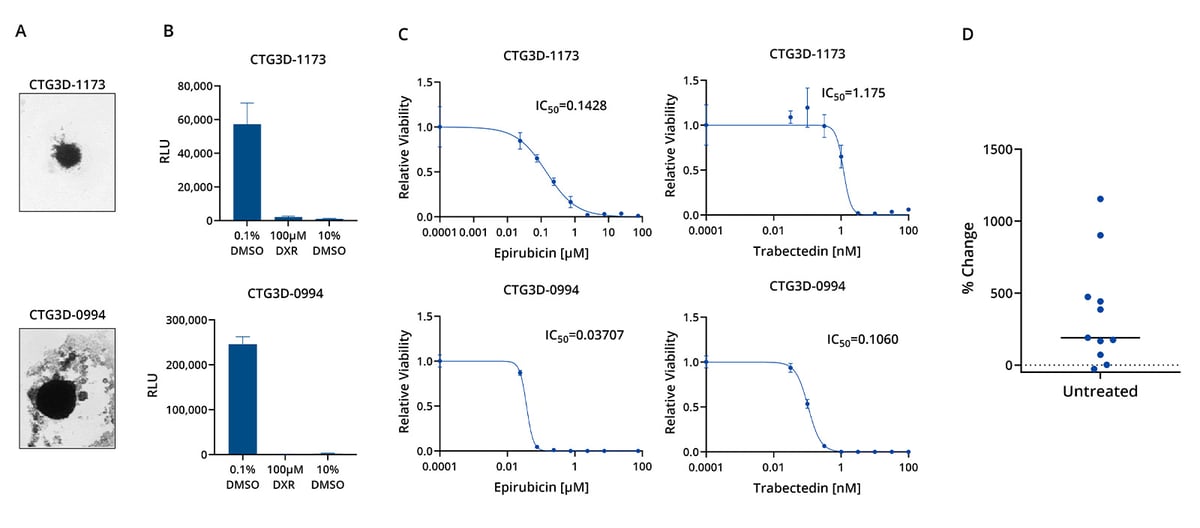
Characterization of Sarcoma TumorGraft3D models CTG3D-1173 and CTG3D-0994: (A) bright field images (4X) confirm 3D structure formation; (B) positive/negative controls prove reliability of the assay; (C) dose response curves show that both models are highly sensitive to epirubicin, while CTG3D-1173 demonstrate a lower sensitivity to trabectedin than CTG3D-0994. (D) Model proliferation during assay duration: models included in the screen are low passage TumorGraft3D derived from low passage PDX models and show a proliferation rate variability that well represents the variability observed in patients.
TumorGraft3D Screen Highlights:
- Screen provides additional cost-saving compared to a stand-alone study
- 11 Sarcoma TumorGraft3D models
- no minimum to enroll
- 50% off of an SOC arm (selected by Champions)
- Included Endpoint: CellTiter-Glo® readout to evaluate cell viability/proliferation with an option to include high-content imaging analysis
- Luminex endpoint analysis can be evaluated at an additional cost
- Custom bioinformatics analyses, or NGS data licensing are available upon request.
