Hematological VitroScreen
May 1st - July 12th, 2024
The industry's largest blood malignancy screen, featuring highly characterized, clinically relevant models of ALL, AML, CLL, MCL, MM, and MDS
Enroll in Champions' industry-leading hematologic model screening platform until July 12th, 2024
Gain access to the largest biobank of extremely difficult-to-source and never passaged before primary hematological malignancies models, including Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL), Multiple Myeloma (MM), and Myelodysplastic Syndrome (MDS) for robust ex vivo investigations. These clinically relevant models offer heterogenous responses, mirroring those observed in patients, ensuring dependable outcomes crucial for advancing your drug discovery efforts with confidence.
Hematological Vitro Screen offers:
- Comprehensive model characterization (stored in Lumin) with:
✅Robust clinical data annotations
✅NGS (RNAseq and WES) analysis
✅Flow Cytometry
✅Proteomics and phospho-proteomics - Largest biobank of difficult-to-source and never passaged (primary) models in the industry.
- Carefully curated models with ex vivo explants and in vivo engraftment capabilities.
- Several endpoint data options available including western blot, flow cytometry, 4D proteomics and more to maximize insights for our partners.
- 50% off of a Standard of Care agent arm (selected by Champions)
- No minimum number of models to enroll and you have the ability to select the models you want to screen.

Figure 1: The Ex Vivo Hematologic Assay workflow outlines the typical study durations for different cancer types and the range of available endpoint data options.
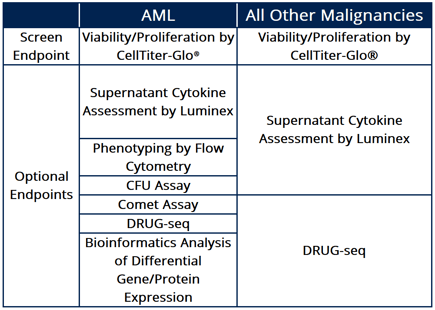
Figure 2: The table displays a variety of endpoint options for different cancer types screened, including AML and other malignancies, utilizing diverse platforms to deliver comparative and reproducible results.

A. CTG-3950 (AML) model responds well to cytarabine and only responds moderately to venetoclax

B. CTG-2406 (ALL) model is responsive to doxorubicin, but not very responsive to cytarabine.



Comet Assay

Figure 3: AML model CTG-3950 shows extensive DNA damage (green tail) after treatment with cisplatin 1μM. Lower doses of cisplatin did not significantly increase DNA damage compared to negative control (0.1% DMSO).
Colony Forming Unit Assay

Figure 4: AML model CTG-2456 shows a prevalence of CFU-GM. Treatment with Cytarabine at doses ≥10nM reduces the formation of these units. ATRA and 10% DMSO were used as positive controls.
